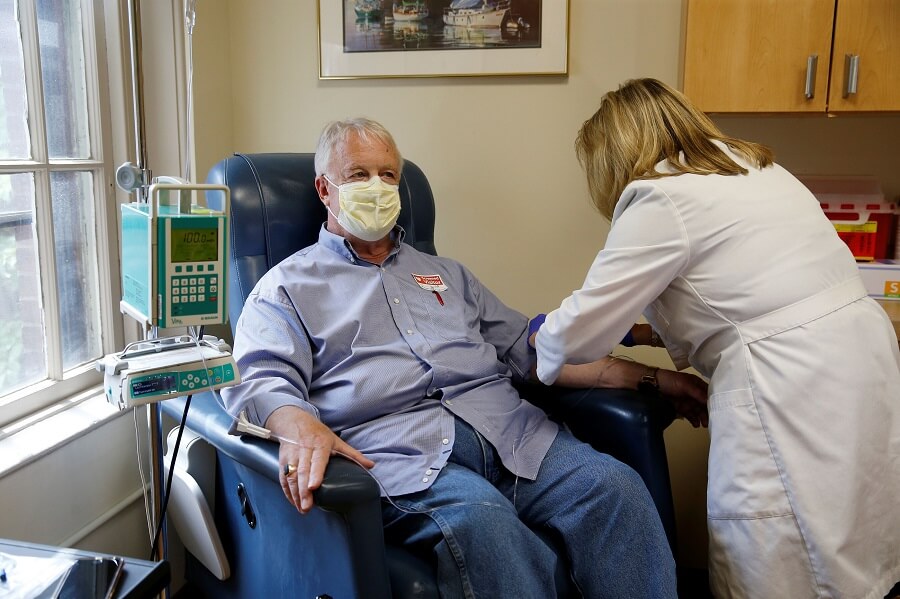
This week, the United States Food and Drug Administration (FDA) approved a new drug to treat Alzheimer’s disease, which had not happened since 2003.
Aducanumab, developed by the biotechnology company Biogen in Cambridge, Massachusetts, is the first approved drug that attempts to treat a possible cause of neurodegenerative disease rather than just the symptoms.
But the FDA approval has sparked a contentious debate over whether the drug is effective. Many experts, including an independent panel of neurologists and biostatisticians, informed the FDA that clinical trial data did not conclusively demonstrate that aducanumab could delay cognitive decline. (2)
In other words, many experts believe that although this drug may seem revolutionary, there are still many steps to take to consider it a cure.
In return, the cost of the annual course of treatment will be $ 56,000. Even The Economist magazine points out that the introduction of the drug into circulation could become a significant burden for insurance companies.
Others worry that the approval of this new drug will have the opposite effect: People with Alzheimer’s could start to drop out of ongoing clinical trials to take aducanumab. Others worry that drug developers may abandon other targets.
“This will set the research community 10 to 20 years back,” says George Perry, a neurobiologist at the University of Texas at San Antonio and skeptical of this new drug and the amyloid hypothesis.
Read on to understand what this new Alzheimer’s drug is about and how it works.
Alzheimer’s Disease: What is it?
Alzheimer’s disease is a neurodegenerative disease first described in 1907 by the German psychiatrist Alois Alzheimer. According to data from 2018, around 52 million people worldwide suffer from this disease, and by 2050 the figure could reach 150 million.
Generally, the disease begins with minor symptoms that progress gradually. First, short-term memory disorders are observed, then long-term memory is disturbed. Infections of speech and cognitive functions occur, and the patient loses the ability to navigate and take care of himself.
In 2000, in the United States, approximately 1.6% of the population had Alzheimer’s disease. In the 75-84 age group, this figure was already 19%, and among citizens older than 84 years, the prevalence of the disease was significant at 42%.
Growing social burden
Alzheimer’s disease is considered the main burden of illness for society in developed countries.
The costs include both direct medical costs (such as nursing homes) and non-medical costs (home care) and indirect costs (lost productivity for both patient and caregiver).
The Amyloid Hypothesis
According to scientific research provided by Biogen, the maker of the new drug Aduhelm, the development of Alzheimer’s disease is associated with the accumulation of a kind of protein plaque in the brain that accumulates between neurons and disrupts their function.
We are talking about the beta-amyloid protein; According to the researchers who developed the new drug, high levels of this substance are associated with impaired cognitive functions of the brain. The new drug can delay the accumulation of protein plaques.
Aducanumab, an antibody infused intravenously, is the latest in many therapeutic candidates that aim to combat amyloid plaques. Although all drugs of this type have so far failed to improve cognition, questions remain as to whether β-amyloid is the correct drug, as well as whether researchers are testing the optimal therapeutic candidates, the proper doses, and the appropriate patients.
Researchers’ concerns now center on the tumultuous passage of aducanumab through clinical trials and the resulting data set, which is incomplete and unpublished.
Post-approval drug testing
As a condition for FDA approval, which was based on the agency’s “accelerated approval” program, Biogen must now conduct a “post-marketing” test to confirm that the drug can improve cognition. It has not yet released details on when and how this test will occur. Biogen has up to nine years to complete the test.
AFTER THE ROLLERCOASTER OF A CLINICAL TRIAL PROGRAM, the FDA’s decision to grant expedited approval to aducanumab could also have broader implications. “This opens the door for pharmaceutical companies looking to use the accelerated approval program to put drugs on the market based on low-quality evidence or a fishery for post-approval data. (2)
Who is the new Alzheimer’s drug for?
Aduhelm is recommended exclusively for people in the early stages of Alzheimer’s disease diagnosed with expensive scans. In practice, a monthly IV cycle is required in a medical facility.
The FDA has separately noted that the registration of a new drug will be withdrawn if the results of clinical trials are not satisfactory. It is important to note that patients need close monitoring, as many develop brain edema.
Forecasts
While there are many questions about the new Alzheimer’s disease drug, according to the Pharmaprojects industry database, there are 148 more drugs in clinical development, with only 15% targeting beta-amyloid.
At the same time, epidemiological studies suggest that some corrective factors (diet, risk of cardiovascular disease, medication, mental activity, and others) are associated with a decrease in the probability of developing the disease.
Intellectual activities (reading, board games, crossword puzzles, playing musical instruments, regular communication) can delay the onset of the disease or mitigate its development. Bilingualism has been associated with a late onset of Alzheimer’s disease.
ABSTRACT
Alzheimer’s disease and dementia remain among the most significant medical, social, and economic problems of the 21st century. Any new drug that can cure (or at least stop) the development of neurodegenerative processes is seen as a breakthrough in science. . However, the approval of drugs without a solid foundation can lead to surprises, even disappointments.